Helicene was recently posted by Molecule of the Day as example of chirality. The post included the link to the Wikipedia page that badly needed updated information. So, when you get to Wikipedia, keep in this in mind…you may miss tons of important stuffs…
Since helicene is one of the two major research projects in our group, we will take on to fill in some information.
Actually, the first racemic [n]helicence, [6]pyrrolohelicene was reported in 1927, followed by the racemic [5]helicence in 1933.
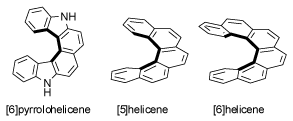 [n]Helicenes structures possess n angularly (ortho) annelated (fused) aromatic rings, in which the steric repulsion of terminal rings force distortion from planarity, leading to helical π-conjugated structures. These molecules possess some of the strongest intrinsic chiral properties. Syntheses of structures with such high degree of ring annelation, and with significant strain are challenging. So, it took a long period of 23 years later before the first successful synthesis of non-racemic [6]helicene was reported by Newman and Lednicer in 1953.
[n]Helicenes structures possess n angularly (ortho) annelated (fused) aromatic rings, in which the steric repulsion of terminal rings force distortion from planarity, leading to helical π-conjugated structures. These molecules possess some of the strongest intrinsic chiral properties. Syntheses of structures with such high degree of ring annelation, and with significant strain are challenging. So, it took a long period of 23 years later before the first successful synthesis of non-racemic [6]helicene was reported by Newman and Lednicer in 1953.
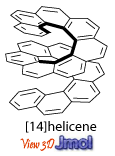
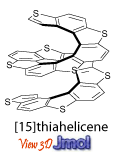 During the 60s and 70s, photochemical syntheses were developed, such method provided numerous [n]helicenes, with [14]helicene as the longest structure consisting of benzene rings. Various [n]helicenes consist of alternating benzene and thiophene rings were also synthesized by the photochemical methods, with the [15]helicene as the longest structure. The photochemical syntheses provided helicenes in low/moderate yield and with limited functionalities.
During the 60s and 70s, photochemical syntheses were developed, such method provided numerous [n]helicenes, with [14]helicene as the longest structure consisting of benzene rings. Various [n]helicenes consist of alternating benzene and thiophene rings were also synthesized by the photochemical methods, with the [15]helicene as the longest structure. The photochemical syntheses provided helicenes in low/moderate yield and with limited functionalities.
Katz group at Columbia have developed very efficient non-photochemical, gram-scale syntheses of functionalized enantiopure [n]helicenes (n = 5, 6, 7).
Recently, two novel classes of helicenes were reported: [n]heliphenes by the Vollhard group at UC-Berkeley and carbon-sulfur [n]helicenes by our group.
More posts will follow…perhaps a series of posts on each class of helicene structures.
- Visit this site to see more cool carbon-sulfur helicene structures.
- Recent review articles to read…
- Andrzej Rajca, Suchada Rajca, Maren Pink, Makoto Miyasaka “Annelated, Chiral π-Conjugated Systems: Tetraphenylenes and Helical -Oligothiophenes”, SynLett, 2007,1799 – 1822 (account, invited) [Abstract].
- Andrzej Rajca, Makoto Miyasaka, “Synthesis and Characterization of Novel Chiral Conjugated Materials”, In Functional Organic Materials – Syntheses and Strategies, T. J. J. Mueller, U. H. F. Bunz, Eds.; Wiley-VCH, 2007, Chapter 15, 543-577 [Download]
- Tomás Torroba, María García-Valverde, “Rigid Annulated Carbon-Sulfur Structures”, Angew. Chem. Int. Ed., 2006, 45, 8092-8096. [Abstract]
- Shawn K. Collins, Martin P. Vachon, “Unlocking the potential of thiaheterohelicenes: chemical synthesis as the key”, Org. Biomol. Chem, 2006, 4, 2518-2524. [Abstract]
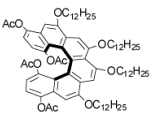
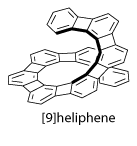
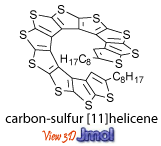


Unfortunately, most of them don’t look soluble at all.
Mitch
LikeLike
Mitch, what do you mean by… most of them *don’t look soluble at all*? Most of them may have very limited solubility, especially those without functional groups. Solubility of the carbon-sulfur [11]helicene was good enough to allow us to obtain C-13 NMR spectra (see SI p.S8-9 of J. Am. Chem. Soc., 127 (40), 13806 -13807, 2005. doi:10.1021/ja055414c). Of course, if we are going to make very long CS-helicene, having two terminal alkyl chains may not be enough. So, we are working on asymmetric synthesis of helicene building blocks that are functionalized with solublizing groups. Visit http://www.chem.unl.edu/rajca/chiral.html#CS to see the structure. We also have prepared a new [7]helicene with more solubilizing groups. The structure is not shown yet, but should be up as soon as we submit the manuscript for publication, hopefully very soon.
LikeLike
Lovely! I do enjoy a good helicene, and those carbon-sulfur helicenes are very cool. Do they have any interesting optical/electronic properties?
LikeLike
Glad to know that you like the post! The annelated beta-oligothiophenes possess a cross-conjugated C-C framework with S atoms at the molecular periphery. X-ray structures show multiple short intermolecular S–S contact. Some of these helicenes form pretty good films. We also determined that the band gap for (C2S)n helix was rather large. We have not done much on the materials testing….though, the OFET measurements were attempted for [7] and [11]helicenes by the groups at Northwestern and at GeorgiaTech, but so far no cigar… We will write another post on carbon-sulfur helicenes soon.
LikeLike
Very interesting to see what a little twist will do–you’ll NEVER see whatever a 14-ringed acene monstrosity would be called.
LikeLike
Ψ*Ψ, do you mean…name for the [14]helicence (C58H32)?
Tetradecahelicene, and the CA index name is Diphenanthro[4,3-g:4′,3′-g’]
naphtho[2,1-c:7,8-c’]diphenanthrene. But who cares…?
LikeLike
Confusion, sorry! I meant it was interesting to compare your twisted structures with the linear acenes I’m used to seeing. Considering the difficulty involved in synthesis of functionalized heptacenes, 14 rings in a linear acene is an impossibility.
How stable are the larger helicenes?
LikeLike
Ψ*Ψ, what is the longest linear acene reported in the literature? Yep, these things are not easy.
Here is a very brief summary about long [n]helicenes.
The [11], [12], and [14]helicenes were reported by Martin and Baes in 1975 (Tetrahedron, 1975, 2135). They were prepared by photochemical syntheses and were characterized by MS, GLC and 1H-NMR, no crystal structure. The helicenes were purified by column chromatography, so they must be quite stable. See also article by Martin and Libert (J. Chem. Research, 1980, 130) for [n]helicenes, n = 8, 9, 10, 11 and 13.
The [n]thiahelicenes (n = 7, 9, 11, 13 and 15) were reported by Yamada et.al, in 1981 (Chem. Lett, 1981, 343), were also prepared by photochemical syntheses, with purification by HPLC, and were characterizated by MS and 1H-NMR. No crystal structure. CD spectrum of [13]thiahelicene enantiomer was shown.
There are lots of examples of [n]helicenes and [n]thiahelicenes, with n = 7 and smaller, prepared by photochemical and non-photochemical syntheses.
Our carbon-sulfur [11]helicene with two n-heptyls was synthesized by iterative, asymmetric synthesis. The [11]helicene was characterized by 1H- and 13C-NMR, MS, UV-vis, CD, CV (cyclic voltammetry) and most importantly the X-ray crystal structure determination. Again, the helicene is quite stable and soluble in organic solvents.
LikeLike
The helicenes are much more soluble than you’d guess from the number of rings. phenanthro[3,4-c[phenanthrene, [5]helicene, is quite soluble in methanol. I think the non-planarity allows more solvent molecules to fit around the structure and that the bent-twistiness makes the pi electrons more interactive than for more planar PAHs.
LikeLike
I have some problem in finding the point group of chiral compounds. what are the basic parameters we needs and is there any sofware of somethings to find out the space arrange ment? please suggest me I am in problem
LikeLike
Look for following symmetry elements in your molecule: (1) plane of symmetry (mirror plane, sigma), (2) center of inversion (i), and (3) improper axis of rotation (Sn). If you find even one of them, then your molecule does not belong to chiral point group. Of course, chiral point groups do not have any of these three elements of symmetry (sigma, I, Sn). You may use GaussView (graphical interface to Gaussian) to check the point group.
LikeLike
Thanks a lot for your suggestion. I got little confused with Bis(4)helicene type of compounds, that i have synthesized recently.
LikeLike
One of my project and your lab project are almost similar. So I am very interested in your blog. Keep it up and please make it up to date.
LikeLike
[…] Ah, Those Aesthetically Pleasing Structures …. [n]Helicenes February 2008 13 comments 4 […]
LikeLike
This is a very informative blog. I am sure chemists will love this. I even learned a lot from here…
LikeLike
Appreciating the time and energy you put into your website and detailed information you offer.
It’s nice to come across a blog every once in a while that isn’t the same
unwanted rehashed material. Great read! I’ve bookmarked your
site and I’m including your RSS feeds to my Google account.
LikeLike