The discovery of organic free radical, the triarylmethyl radical, was reported in JACS by Moses Gomberg in 1900.1 The report ended with an interesting statement.

“This work will be continued”—that is great! But “and I wish to reserve the field for myself”—what… and why? Think about this—that was way before the birth of the World Wide Web—no bloggers, twitters, etc. Just imagine what would happen these days—would such statement get through the reviewers and editors? Likely, no, but who knows, many odd things have shown up in the literature lately. Good thing though, that was ignored and the field has advanced.
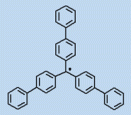 Credited to Wilhelm Schlenk who provided definitive evidence for the free radical concept.2 In 1910, Schlenk prepared tris(biphenyl)methyl radical and isolated it as black crystals. In solution, this radical was almost completely monomeric. The result removed all doubt concerning the existence of the radical.
Credited to Wilhelm Schlenk who provided definitive evidence for the free radical concept.2 In 1910, Schlenk prepared tris(biphenyl)methyl radical and isolated it as black crystals. In solution, this radical was almost completely monomeric. The result removed all doubt concerning the existence of the radical.
Five years later, Schlenk and Max Brauns reported the first organic diradical, which was prepared by metal reduction of the corresponding dichloride.3 However, there were large amounts of monoradical and perhaps oligomers/polymers as side products.
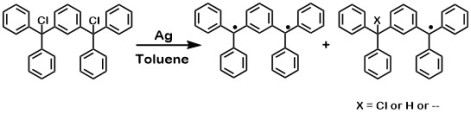
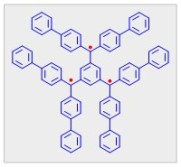 In 1937, Martin Leo attempted preparation of organic triradical by treating trichloride with Cu. However, the product was not characterized.4 It took another 28 years before a slightly more stabilized triradical, 1,3,5-tris(di-p-biphenylmethyl)benzene was reported in 1965 by Schmauss, Baumgartel, and Zimmermann.5 Once again, because the triradical was prone to rapid dimerization, its characterization was not established until 1971.6
In 1937, Martin Leo attempted preparation of organic triradical by treating trichloride with Cu. However, the product was not characterized.4 It took another 28 years before a slightly more stabilized triradical, 1,3,5-tris(di-p-biphenylmethyl)benzene was reported in 1965 by Schmauss, Baumgartel, and Zimmermann.5 Once again, because the triradical was prone to rapid dimerization, its characterization was not established until 1971.6
In 1988, the Rajca Lab was “born” at Kansas State University in Manhattan, Kansas. One of the two research projects was organic magnets—quite an ambitious goal! The target molecules were “planarized” 1,3-connected polyarylmethyls. Synthesis of such annelated structures was evidently not an easy task. There was a bright new student ready to join the group and the student was more interested in physical chemistry than organic synthesis. It would have been too much for the student to jump into difficult synthesis from the beginning. Thus, a simple synthetic scheme of a star-branched polyarylmethyl was drawn upon. Here is the synthetic scheme.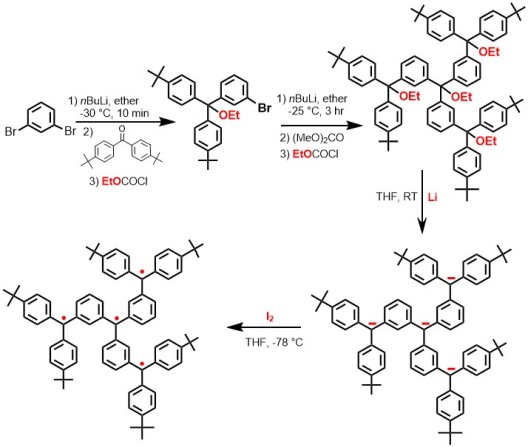
However, it turned out that the student was not allowed to join the group early, and later changed his mind to join the physical chemistry group. This was a big blow! The young assistant professor decided to get his hands dirty and started the synthesis by himself. Two years later, JACS communications were published back-to-back, to demonstrate an efficient method for generation of carbopolyanions (carbanion method)7 which provided the intermediates that were cleanly converted to polyradicals.8, This accomplishment laid a solid foundation for the Rajca research group. High spin polyarylmethyl polyradicals became the main project and numerous high spin polyarylmethyl polyradicals have been prepared since then.
The circumstance has always been remembered for the positive outcome and an important step. If things were different—the student was interested in organic synthesis, the simple synthetic scheme of the star-branched polyarylmethyl might have not been drawn up or the project just disappeared with the student—the Rajca lab might not have survived. It turned out the “planarized” 1,3-connected polyarylmethyls did not go far beyond a stable diradical! Tremendous investment in the syntheses of the precursor to the tetraradical and the larger homologous just did not pay off in the end.
A lot of things we encounter in doing research can help developing positive mindset. How failures are handled is as important as the success. Epictetus tells us, “Everything has two handles, the one by which it may be carried, the other by which it cannot. If your brother acts unjustly, don’t lay hold on the action by the handle of his injustice, for by that it cannot be carried; but by the opposite, that he is your brother, that he was brought up with you; and thus you will lay hold on it, as it is to be carried”. Marietta McCarty put it more clearly in “Choosing the Glass Half Full”
Up next…. how much further we made it with the star-branched polyarylmethyl and how we handled the fatal failure.
1. M. Gomberg, “An Instance of Trivalent Carbon: Triphenylmethyl’’, J. Am. Chem. Soc, 1900, 22, 757 doi:10.1021/ja02049a006 (The Triphenylmethyl Radical and Triphenylmethyl radical: properties and synthesis).
2. Thomas T. Tidwell, “Wilhelm Schlenk: The Man Behind the Flask”, Angew. Chem. Int. Ed., 2001, 40, 331 doi:10.1002/1521-3773(20010119)40:2<331::AID-ANIE331>3.0.CO;2-E (pdf)
3. W. Schlenk and M. Brauns, Chem. Ber., 1915, 48, 661 doi:10.1002/cber.19150480189
4. M. Leo, “Uber Radikale mit mehreren dreiwertigen Kohlenstoffatomen”, Ber., 1937, 70, 1691.
5. G. Schmauss, H. Baumgartel, and H. Zimmermann, “1,3,5-Tris(di-p-biphenylmethyl)benzene, a New Triradical”, Angew. Chem. Int. Ed., 1965, 4, 596 doi:10.1002/anie.196505962
6. G. Kothe, E. Ohmes, J.Brickmann, and H. Zimmermann, “1,3,5-Benzenetriyltris[di(p-biphenyl)methyl], a Radical Having a Quartet Ground State that Dimerizes by Entropy”, Angew. Chem. Int. Ed., 1971, 10, 938 doi:10.1002/anie.197109381
7. A. Rajca, “A Polyarylmethyl Carbotetraanion”, J. Am. Chem. Soc., 1990, 112, 5889. doi:10.1021/ja00171a044
8. A. Rajca, “A Polyarylmethyl Quintet Tetraradical”, J. Am. Chem. Soc., 1990, 112, 5890. doi:10.1021/ja00171a045


 Credited to Wilhelm Schlenk who provided definitive evidence for the free radical concept.2 In 1910, Schlenk prepared tris(biphenyl)methyl radical and isolated it as black crystals. In solution, this radical was almost completely monomeric. The result removed all doubt concerning the existence of the radical.
Credited to Wilhelm Schlenk who provided definitive evidence for the free radical concept.2 In 1910, Schlenk prepared tris(biphenyl)methyl radical and isolated it as black crystals. In solution, this radical was almost completely monomeric. The result removed all doubt concerning the existence of the radical.
 In 1937, Martin Leo attempted preparation of organic triradical by treating trichloride with Cu. However, the product was not characterized.4 It took another 28 years before a slightly more stabilized triradical, 1,3,5-tris(di-p-biphenylmethyl)benzene was reported in 1965 by Schmauss, Baumgartel, and Zimmermann.5 Once again, because the triradical was prone to rapid dimerization, its characterization was not established until 1971.6
In 1937, Martin Leo attempted preparation of organic triradical by treating trichloride with Cu. However, the product was not characterized.4 It took another 28 years before a slightly more stabilized triradical, 1,3,5-tris(di-p-biphenylmethyl)benzene was reported in 1965 by Schmauss, Baumgartel, and Zimmermann.5 Once again, because the triradical was prone to rapid dimerization, its characterization was not established until 1971.6
![carbon-sulfur [15]helicene retrosynthesis carbon-sulfur [15]helicene retrosynthesis](https://rajcalab.files.wordpress.com/2010/01/cs15_retro2.gif?w=605)
![Bis[7]helicenes Bis[7]helicenes](https://rajcalab.files.wordpress.com/2010/01/bishelicene_struc1.gif?w=605)
![Bis[7]helicene Xray structure Bis[7]helicene Xray structure](https://rajcalab.files.wordpress.com/2010/01/bis7helicene_2-tps2_xray.gif?w=605)